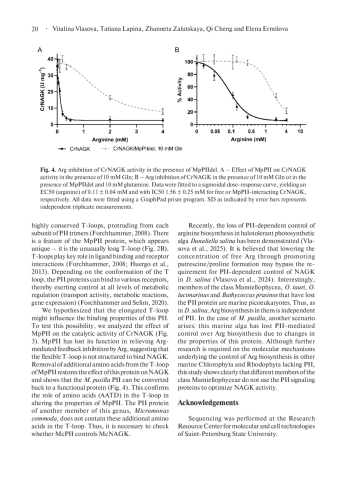
The PII superfamily consists of widespread signal transduction proteins found in all domains of life. The most conserved PII-interactor across oxygenic phototrophs from cyanobacteria to Archaeplastida is the key enzyme of the ornithine/arginine synthesis pathway, N-acetyl-L-glutamate kinase (NAGK). T-loops represent the major PII-receptor binding element and are involved in the interaction with NAGK. Within the class Mamiellophyceae, only the genus Micromonas contains species with the PII protein. Bioinformatic analysis revealed that the PII protein of Micromonas pusilla (MpPII) has an unusually prolonged T-loop. Here, we performed the coupled enzyme assay and showed that MpPII has no remarkable influence on NAGK activity. An engineered variant of MpPII with deletion of four additional amino acids (AATD) in the T-loop restored the ability of this protein to relieve NAGK from feedback inhibition by arginine in a glutamine-dependent manner. The findings are discussed in the context of unusual plasticity of the PII protein family during the evolution of Archaeplastida.
Идентификаторы и классификаторы
- SCI
- Биология
The PII proteins are highly conserved and widely distributed signal transduction proteins known in all domains of life (Fokina et al., 2010; Huergo et al., 2013; Forchhammer and Selim, 2020; Selim et al., 2020a). However, in the eukaryotes, the PII homologs inherited from a cyanobacterial endosymbiont are restricted to Archaeplastida (Chellamuthu et al., 2013).
Список литературы
1. Beez S., Fokina O., Herrmann C. and Forchhammer K. 2009. N-acetyl-L-glutamate kinase (NAGK) from oxygenic phototrophs: PII signal transduction across domains of life reveals novel insights in NAGK control. J. Mol. Biol. 389 (4): 748- 758. DOI: 10.1016/j.jmb.2009.04.053
2. Beyer H.M., Gonschorek P., Samodelov S.L., Meier M. et al. 2015. AQUA Cloning: A versatile and simple enzyme-free cloning approach. PLoS One. 10 (9): e0137652. DOI: 10.1371/journal.pone.0137652
3. Blanc-Mathieu R., Verhelst B., Derelle E., Rombauts S. et al. 2014. An improved genome of the model marine alga Ostreococcus tauri unfolds by assessing Illumina de novo assemblies. BMC Genomics. 15 (1):1103. DOI: 10.1186/1471-2164-15-1103
4. Bueno R., Pahel G. and Magasanik B. 1985. Role of glnB and glnD gene products in regulation of the glnALG operon of Escherichia coli. J. Bacteriol. 164 (2): 816-822. DOI: 10.1128/jb.164.2.816-822.1985
5. Chellamuthu V.R., Alva V. and Forchhammer K. 2013. From cyanobacteria to plants: Conservation of PII functions during plastid evolution. Planta. 237 (2): 451-462. DOI: 10.1007/s00425-012-1801-0
6. Chellamuthu V.R., Ermilova E., Lapina T., Lüddecke J. et al. 2014. A widespread glutamine-sensing mechanism in the plant kingdom. Cell. 159 (5): 1188-1199. DOI: 10.1016/j.cell.2014.10.015
7. Chen Y.M., Ferrar T.S., Lohmeier-Vogel E.M., Morrice N. et al. 2006. The PII signal transduction protein of Arabidopsis thaliana forms an arginine regulated complex with plastid N-acetyl glutamate kinase. J. Biol. Chem. 281 (9): 5726-5733. DOI: 10.1074/jbc.M510945200
8. Fokina O., Chellamuthu V.R., Forchhammer K. and Zeth K. 2010. Mechanism of 2-oxoglutarate signaling by the Synechococcus elongatus PII signal transduction protein. Proc. Natl. Acad. Sci. USA. 107 (46): 19760-19765. DOI: 10.1073/pnas.1007653107
9. Forcada-Nadal A., Llácer J.L., Contreras A., Marco-Marín C. et al. 2018. The PII-NAGK- PipX-NtcA regulatory axis of Cyanobacteria: A tale of changing partners, allosteric effectors and non-covalent interactions. Front. Mol. Biosci. 5:91. DOI: 10.3389/fmolb.2018.00091
10. Forchhammer K. 2008. PII signal transducers: novel functional and structural insights. Trends Microbiol. 16 (2): 65-72. DOI: 10.1016/j.tim.2007.11.004
11. Forchhammer K. and Selim K.A. 2020. Carbon/ nitrogen homeostasis control in cyanobacteria. FEMS Microbiol. Rev. 44 (1): 33-53. DOI: 10.1093/femsre/fuz025
12. Guo X., Pang M., Zheng X. and Huang L. 2024. Micromonas, a small pigmented flagellate, predominates the nanoflagellate and photosynthetic picoeukaryote communities in the northern South China Sea. Environ. Microbiol. Rep. 16 (2): e13244. DOI: 10.1111/1758-2229.13244
13. Heinrich A., Maheswaran M., Ruppert U. and Forchhammer K. 2004. The Synechococcus elongatus PII signal transduction protein controls arginine synthesis by complex formation with N- acetylL-glutamate kinase. Mol. Microbiol. 52 (5): 1303-1314. DOI: 10.1111/j.1365-2958.2004.04058.x
14. Huergo L.F., Chandra G. and Merrick M. 2013. P(II) signal transduction proteins: nitrogen regulation and beyond. FEMS Microbiol. Rev. 37 (2): 251-83. DOI: 10.1111/j.1574-6976.2012.00351.x
15. Lapina T., Selim K.A., Forchhammer K. and Ermilova E. 2018. The PII signaling protein from red algae represents an evolutionary link between cyanobacterial and Chloroplastida PII proteins. Sci. Rep. 8 (1): 790. DOI: 10.1038/s41598-017-19046-7
16. Leliaert F., Verbruggen H. and Zechman F.W. 2011.Into the deep: New discoveries at the base of the green plant phylogeny. Bioessays. 33 (9): 683-692. DOI: 10.1002/bies.201100035
17. Llácer J.L., Contreras A., Forchhammer K., Marco-Marín C. et al. 2007. The crystal structure of the complex of PII and acetylglutamate kinase reveals how PII controls the storage of nitrogen as arginine. Proc. Natl. Acad. Sci. USA. 104 (45): 17644-17649. DOI: 10.1073/pnas.0705987104
18. Llácer J.L., Fita I. and Rubio V. 2008. Arginine and nitrogen storage. Curr. Opin. Struct. Biol. 18 (6): 673-681. DOI: 10.1016/j.sbi.2008.11.002
19. Maheswaran M., Urbanke C. and Forchhammer K. 2004.Complex formation and catalytic activation by the PII signaling protein of N-acetyl-L-glutamate kinase from Synechococcus elongatus strain PCC 7942. J. Biol. Chem. 279 (53): 55202-55210. DOI: 10.1074/jbc.M410971200
20. Marin B. and Melkonian M. 2010. Molecular phylogeny and classification of the Mamiellophyceae class. nov. (Chlorophyta) based on sequence comparisons of the nuclear- and plastid-encoded rRNA operons. Protist. 161 (2): 304-336. DOI: 10.1016/j.protis.2009.10.002
21. Mizuno Y., Moorhead G.B. and Ng K.K. 2007. Structural basis for the regulation of N-acetyl-glutamate kinase by PII in Arabidopsis thaliana. J. Biol. Chem. 282 (49): 35733-35740. DOI: 10.1074/jbc.M707127200
22. Moreau H., Verhelst B., Couloux A., Derelle E. et al. 2012. Gene functionalities and genome structure in Bathycoccus prasinos reflect cellular specializations at the base of the green lineage. Genome Biology. 13. R74. DOI: 10.1186/gb-2012-13-8-r74
23. Sant’Anna F.H., Trentini D.B., Weber S.S., Cecagno R. et al. 2009. The PII superfamily revised: a novel group and evolutionary insights. J. Mol. Evol. 68 (4): 322-336. DOI: 10.1007/s00239-009-9209-6
24. Selim K.A., Ermilova E. and Forchhammer K. 2020a. From cyanobacteria to Archaeplastida: New evolutionary insights into PII signalling in the plant kingdom. New Phytol. 227 (3): 722-731. DOI: 10.1111/nph.16492
25. Selim K.A., Lapina T., Forchhammer K. and Ermilova E. 2020b.Interaction of N-acetyl-l-glutamate kinase with the PII signal transducer in the non-photosynthetic alga Polytomella parva: co-evolution towards a hetero-oligomeric enzyme. FEBS J. 287 (3): 465-482. DOI: 10.1111/febs.14989
26. Sugiyama K., Hayakawa T., Kudo T., Ito T. and Yamaya T. 2004.Interaction of N-acetylglutamate kinase with a PII-like protein in rice. Plant Cell Physiol. 45 (12): 1768-1778. DOI: 10.1093/pcp/pch199
27. Truan D., Huergo L.F., Chubatsu L.S., Merrick M. et al. 2010. A new PII protein structure identifies the 2-oxoglutarate binding site. J. Mol. Biol. 400 (3): 531-539. DOI: 10.1016/j.jmb.2010.05.036
28. Vlasova V., Lapina T., Cheng Q. and Ermilova E. 2025. Loss of PII-dependent control of arginine biosynthesis in Dunaliella salina. Plant Sci. 351. 112327. DOI: 10.1016/j.plantsci.2024.112327
29. Vlasova V., Lapina T., Zalutskaya Z., Puzanskiy R. et al. 2024. Control of arginine biosynthesis in the green alga Dunaliella salina. Protistology. 18 (3): 212-220. DOI: 10.21685/1680-0826-2024-18-3-3
30. Worden A.Z., Lee J.H., Mock T., Rouzé P. et al. 2009. Green evolution and dynamic adaptations revealed by genomes of the marine picoeukaryotes Micromonas. Science. 324 (5924): 268-272. DOI: 10.1126/science.1167222
31. Zeth K., Fokina O. and Forchhammer K. 2014. Structural basis and Target-Specific modulation of ADP Sensing by the Synechococcus elongatus PII signaling protein. J. Biol. Chem. 289: 8960-72.
Выпуск
Другие статьи выпуска
Thecamoeba onigiri n. sp. (Amoebozoa, Discosea, Thecamoebida) was isolated from a moss sample collected in the surroundings of Lake Baikal (Russia). The amoebae of this species belong to the striate morphotype and have a single rounded nucleus. The nucleolar material of T. onigiri can be organized in different ways depending on the age of the culture. The most common was a nucleus with a nearly spherical eccentric nucleolus, sometimes located very close to the nuclear envelope. The surface of such a nucleolus was rough and uneven. This type of nucleolar organization was observed in one- to two-week-old cultures. In contrast, a centrally located rounded nucleolus with smooth surface predominated in three- to four-week-old cultures. This type of the nucleolus corresponds to the classical “vesicular” nucleus and is similar to that found in amoebae of “T. quadrilineata species group”, but differs in having lacunae that are more peripheral. Molecular phylogenetic analysis based on 18S rRNA gene sequences showed that T. onigiri forms a clade with T. astrologa as part of a larger group that also includes the “T. quadrilineata species group” and T. aesculea. Meanwhile, two species of Thecamoeba demonstrate polymorphism of the nucleolar material arrangement, and both these species are phylogenetically close.
Protists inhabit waters with different salinities up to saturation, and play significant roles in the food webs. In hypersaline environments, the simplified communities are composed of mainly pro- and eukaryotic halophilic microorganisms. The aim of this study was to characterize the prokaryotic assemblages associated with uniprotist cultures of heterotrophic and phototrophic halophilic protists isolated from inland saline water bodies and maintained under laboratory conditions for a long time. The cultures were represented by chlorophycean algae Dunaliella and Asteromonas, as well as by heterotrophic protozoa of the phylum Heterolobosea. DNA metabarcoding revealed that the prokaryotic assemblages differed drastically between the phototrophic and heterotrophic protists in richness, diversity and taxonomic composition. Generally, the prokaryotic assemblages of Heterolobosea protozoa were richer than those of chlorophycean algae. In the algal microbiomes, only few prokaryotic genera were revealed. They were represented by the bacterial phylum Pseudomonadota and the archaeal phylum Halobacteriota. Rather diverse prokaryotic assemblages were associated with the cultures of halophilic Heterolobosea protozoa. They were composed of the phyla Pseudomonadota, Bacteroidota, Balneolota, Thermodesulfobacteriota, Halobacteriota, and Nanohaloarchaeota. Predicted functional profiles of the prokaryotic assemblages revealed the pathways responsible for close metabolic interactions between the halophilic protists and their prokaryotic associates, including synthesis of organic osmotic solutes and their conversion, degradation of sugar and organic aromatic compounds, biosynthesis of cofactors and vitamins. The dramatic differences between prokaryotic assemblages of heterotrophic and phototrophic protists suggest the existence of different selection mechanisms shaping microbiomes of the halophilic protists that still have to be elucidated.
Although the global distribution of eumycetozoans in terrestrial ecosystems with varying vegetation types has been a subject of a number of investigations during the past decade, there is still scarce to no available data from the mangrove forests, particularly in the Philippines. Hence, this study assesses and compares the occurrence and distribution of protosteloid amoebae inhabiting the mangrove ecosystem of San Fernando City, La Union, with the villages Biday and Catbangen as representatives. Aerial (AL) and ground (GL) leaf litter samples were used as substrates in isolating protosteloid amoebae and were subjected to moist chamber cultures. The 17 species belonging to 12 genera, from a total of 125 records, were described and reported in this study, with Protostelium mycophagum being the most often occurring species. Further results indicate that the ground microhabitat and the Biday site exhibited higher species diversity and abundance than the aerial microhabitat and the Catbangen site. Regarding species richness in the two leaf litters, GL hosted higher species richness than AL. The current research is one of the few that has assessed and surveyed the eumycetozoan distribution, occurrence, and ecology in the Philippine mangrove ecosystem. Furthermore, it demonstrates the potential for a mangrove forest to support diverse protosteloid amoebae growth.
In recent decades, infection of the European honey bee Apis mellifera with the highly virulent microsporidium Vairimorpha (Nosema) ceranae has become globally prevalent. It causes serious losses in beekeeping worldwide and requires new approaches to control bee nosemosis. Since this intracellular parasite has retained components of the RNA interference pathway, double-stranded RNA (dsRNA) treatment of insects may be effective in controlling V. ceranae infection. Inhibition of microsporidia growth in bees fed with 1.8 or even 0.04 μg dsRNA per ml of sugar syrup has been reported in the literature. Considering the crucial role of the genome’s DNA replication machinery for any cell, we synthetized in vitro dsRNA fragments of four V. ceranae genes encoding two subunits (delta and epsilon) of DNA polymerase, helicase and topoisomerase II and fed them to the infected bees with a relatively low dose of 1 µg per ml of sugar syrup. Surprisingly, PCR and qPCR analyses of V. ceranae growth in the midgut of dsRNA-treated insects at 7- and 12-days post-infection revealed neither inhibition of microsporidia growth nor downregulation of target genes. It is worth noting that we collected worker bees of different ages directly from a hive, to simulate the conditions during colony treatment in apiaries. At the same time, newly emerged insects reared in the laboratory were used in the successful experiments mentioned above. This suggests that bee housing conditions as well as gut content may affect the efficiency of RNA interference and requires further increase in dsRNA doses or their mixing with nanoparticle carriers.
Статистика статьи
Статистика просмотров за 2025 год.
Издательство
- Издательство
- ИНЦ
- Регион
- Россия, Санкт-Петербург
- Почтовый адрес
- Санкт-Петербург, 194064, Тихорецкий проспект, 4
- Юр. адрес
- Санкт-Петербург, 194064, Тихорецкий проспект, 4
- ФИО
- Томилин Алексей Николаевич (Руководитель)
- E-mail адрес
- cellbio@incras.ru
- Контактный телефон
- +7 (812) 2971878
- Сайт
- https:/incras.ru